Understanding Evaporation
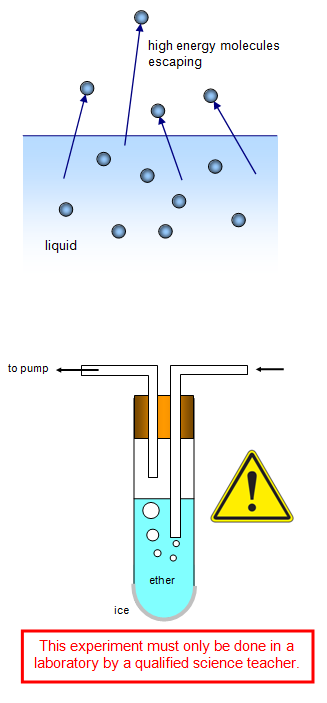
Evaporation happens when a liquid becomes a gas. It’s happening all around you! Think of a puddle drying after the rain. That’s evaporation in action!Volatility: A Key Player
Perfumes are made of substances that are naturally “volatile”. This means they love to become a gas. Water, on the other hand, is less volatile. It likes being a liquid more than a gas.Molecules on the Move
Imagine a dance party, where molecules are the dancers. Perfume molecules dance away into the air fast! Water molecules are the slow dancers, taking their time before joining the air.Ingredient Differences
- Perfumes contain special ingredients called “essential oils”.
- Water is just, well, water – H2O.
Boiling Points
Boiling points are like a molecule’s fever temperature. When they reach a certain hotness, they turn into gas. Perfume’s boiling point is lower than water. So, it turns into gas quicker than water.| Liquid | Boiling Point |
|---|---|
| Perfume (Essential Oil) | Lower |
| Water (H2O) | Higher |
It’s All in the Smell
Why do we wear perfume? For the delightful smell! Perfume makers design the scent to spread and be noticed. If it stayed put like water, you wouldn’t smell as sweet.The Power of Heat
Your body gives off warmth. Just like how ice cream melts in the sun, your body heat helps perfume evaporate.Surface Area: Spreading Out
When you spray perfume, it creates a mist. More surface area is exposed to air. This helps it to evaporate even faster than if it were just a blob.Concentration and Composition
Perfumes often have alcohol. Alcohol evaporates really fast. This helps carry the scent into the air. Water doesn’t have these speedy helpers. Quick Tips to Make Perfume Last Longer:- Apply right after a shower.
- Don’t rub it in, let it dry naturally.
- Store it in a cool, dark place.













